
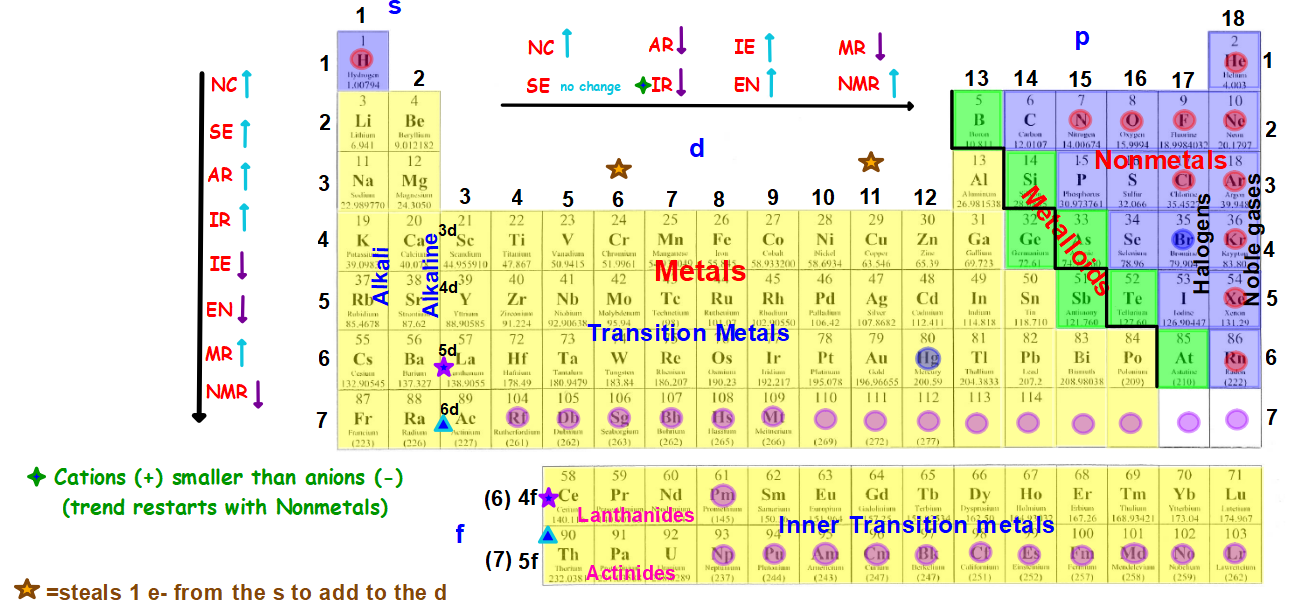
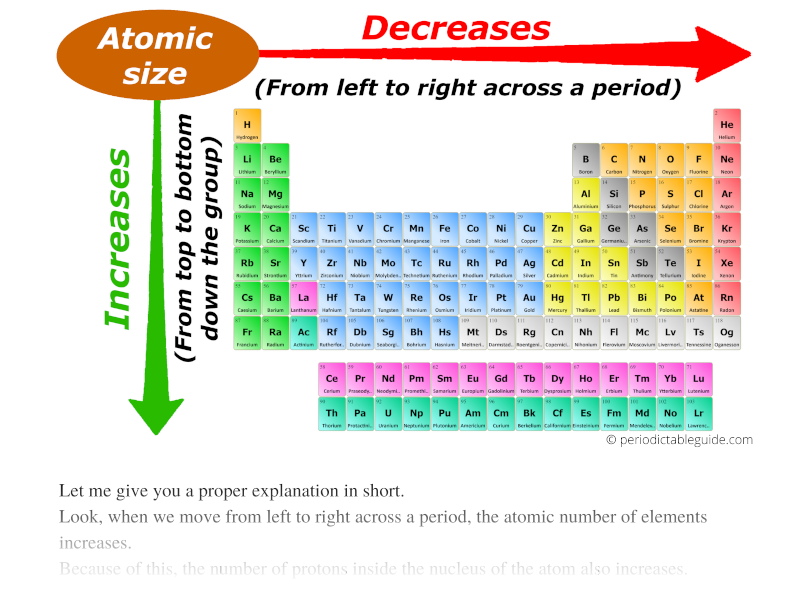
In lanthanoids, the atomic and ionic radii decrease with increase in atomic number. Lanthanum is the first element and Lutetium is the last one. Lanthanum, La and Lutetium, Lu belong to lanthanoids (4f block elements of inner transition elements). Which one of the following given values will be closest to the radius of Lu 3+ (atomic number : Lu =71) ? 6) The radius of La 3+ (atomic number : La=57) is 1.06 Å. The correct order of ionic radii is Na + > Mg 2+ > Al 3+ > Si 4+ as given in option "4". The correct option is "d" 5) Which of the following is correct order of ionic radii?įor isoelectronic ions, the radius decreases with increase in positive charge. For different ions of same element, size decreases with increase in the positive charge. K + and Cl - are isoelectronic species but the number of protons are more in K +. Since the proton number is same in isotopes, the nuclear attraction is also same. These ions belong to two different isotopes of same element. Chemistry Classification of Elements and Periodicity in Properties Periodic Trends Of Ionic Radii In Modern Periodic Table Ionic Radius Ionic radii, what is it Let’s figure out what we mean by ionic radii and their variation in groups and periods of the periodic table ( modern eriodic table ). The size decrease with increase in the positive charge these species. The atomic size increases from top to bottom in a group. Size decreases with increase in proton number The ionic radii increases with decrease in the effective nuclear charge. Generalization: In iso-electronic species, the atomic size decreases with increase in the atomic number. Hence the attraction is minimum and the ion is largest. However, in N 3- ion, there is only 0.7 proton for each electron. Hence the attraction is maximum and the ion is smallest among the given species. Greater this value, greater is the attraction and smaller is the size.įor example, in Na + ion, there is 1.1 proton for each electron. of protons (Z) to number of electrons correlates effective nuclear attraction. Related questions 2) Which of the following is the smallest in size? Note: It is not possible to get covalent and metallic radii for noble gases since they do not form bonds.Ībove fact is reflected in option "a". Hence neon's atomic radius must be much more than that of fluorine. The reported radii of noble gas elements are "van der Waals radii", which are 40% more than the actual atomic radii. PERIODIC TRENDS IN RADIUS OF ATOM AND IONĪdiChemistry IIT JEE Atomic radii of fluorine and neon in Angstrom units are respectively given by: Questions periodic trends | atomic radius | IIT JEE | NEET


 0 kommentar(er)
0 kommentar(er)
